2 Results and Discussion
2.1 Emulsifiers
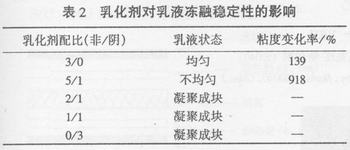
The emulsifier is adsorbed on the surface of latex particles in the emulsion system to form an adsorbent protective layer to stabilize it. As can be seen from Table 2, when all non-ionic emulsifiers are used in the system, the emulsion freezes and thawing is uniform, delicate, still fluid, the viscosity change rate is relatively small, and has strong freeze-thaw resistance; when the system is added When the anionic emulsifier is used, the emulsion is not uniform after one freezing and thawing, and the viscosity sharply increases, but it can be restored to a uniform emulsion after stirring; when the content is > 1/3, the emulsion is condensed into blocks after freezing and thawing for one time. Reduced melt stability and poor resistance to freezing and thawing.
2.2 Buffers
2.2.1 Kinds of buffers
In the polymerization process, the acid produced by the initiator causes the pH value of the system to decrease. The pH value of the system affects the decomposition rate of the initiator and the hydrolysis rate of the monomer. The addition of a buffer can adjust the pH of the system to ensure the pH value of the polymerization system is stable. The polymerization reaction is stable; at the same time, the buffer is also an electrolyte, which has a great influence on the performance of the emulsion [4]. Therefore, different kinds of buffers have different effects on the stability of emulsions in different emulsion systems and the concentration of buffers.
Select NaHCO3-NaAc, Na2HPO4 as a buffering agent. The results are shown in Table 3.

It can be seen from Table 3 that when PVA1788/1799 is selected as the protective colloid, when NaHCO3 is added, coarse particles appear after the emulsion is freeze-thawed once; when NaAc is added, the appearance of the emulsion is uneven, and the emulsion can be restored to uniform emulsion after being stirred; Na2HPO4 was added and the emulsion was emulsion-agglomerated after 1 freeze-thaw cycles. Therefore, when PVA1788/1799=1/1 is a protective colloid, NaAc is selected as a buffer, and the emulsion freeze-thaw is relatively stable. In the protective colloid emulsion PVA1788, Na2HPO4 is added as a buffer, the emulsion can be restored to a homogeneous emulsion after one freeze-thaw; however, the viscosity change rate of NaHCO3, NaAc is relatively large, and the appearance of the emulsion can be restored to uniform after stirring. Emulsion. Therefore, when PVA1788 is used as a protective colloid, Na2HPO4 is selected as a buffer, and the emulsion freeze-thaw stability is good. During the seed preparation process, the pH values ​​of the emulsions were all measured from 6.0 to 6.5. This indicates that the buffering agents have basically the same adjustment effect on the pH of the system. The difference in emulsion freeze-thaw stability is due to different types of ions causing emulsions. Double layer structure caused by changes.
Therefore, different types of buffers have different effects on the emulsion freeze-thaw stability in different protective colloid systems.
2.2.2 Buffer Concentration
With the change of the buffer concentration of the system, the pH value of the emulsion did not change much, but the electrolyte concentration in the system changed. Table 4 shows that the protective colloid is PVA 1788/1799=1/1, NaAc is a buffer, and its concentration has an effect on the stability of the emulsion. When the buffer concentration is 0.1%, the viscosity change rate of the emulsion after freezing and thawing is larger; when the concentration is 0.2%, the emulsion can be recovered after freezing and thawing; when the concentration is 0.5%, the emulsion is not frozen and thawed Even emulsion, but the rate of change of viscosity is small. Therefore, the concentration of electrolyte should not be too large, exceeding a certain range is not beneficial to the freeze-thaw stability of the emulsion. When the protective colloid is PVA1788 and Na2HPO4 is a buffer, its concentration has an effect on the stability of the emulsion. When the concentration is 0.5%, the emulsion can be restored to a homogenous state with stirring after one freezing and thawing, and the viscosity change rate is large; when the concentration is increased to 1.0%, the emulsion can be restored to a uniform state after freezing and thawing. The viscosity change rate was 17%. When the concentration is 2.0%, the viscosity change rate of the emulsion after 1 freeze-thaw cycle is 40%, and the appearance is still uniform emulsion.

(to be continued)

