Waste carton wall hanging

Waste carton wall hangings change

Description about Coronavirus(COVID-19)test Test
The Coronavirus(COVID-19)test is a solid phase immunochromatographic assay used in the rapid, qualitative and differential detection of IgG and IgM antibodies to the 2019 novel coronavirus in human whole blood, serum or plasma. The Coronavirus(COVID-19)test is intended for professionals within highly complex settings and or pharmacies. The test delivers rapid results between 2 and 10 minutes from individuals having signs and symptoms of SARS-CoV-2 infection
What conditions to do the Coronavirus(COVID-19)test?
1.Breating difficulty
2.skin sores, which may become a skin ulcer that heals very slowly
3.stuffy nose, runny nose, and nosebledds.
4.swallowing difficulty
TEST OVERVIEW for the Coronavirus(COVID-19)test
- Utilizes human whole blood, serum, or plasma
- Used in rapid, qualitative and differential detection of IgG and IgM antibodies
- Delivers clinical results between 2 and 10 minutes at the point-of-care
- Visual interpretation of results
- No special equipment needed
PERFORMANCE CHARACTERISTICS
The COVID-19 lgG/lgM Rapid Test (Whole Blood/Serum/Plasma) has been evaluated with the 126 samples obtained from patients exhibiting pneumonia or respiratory symptoms. The results were compared to Fluorescent Real Time Polymerase Chain Reaction (RT-PCR) or clinical diagnosis (including chest Computed Tomography and clinical signs etc.) of [Diagnosis and treatment of novel coronavirus pneumonia."
IgG/IgM Rapid Test results compared to SARS-CoV-2 RT-PCR results:
| COVID-19 RT-PCR Assay | Coronavirus(COVID-19)test | ||
| Positive | Negative | Total | |
| Positive | 103 | 9 | 112 |
| Negative | 0 | 14 | 14 |
| Total | 103 | 23 | 126 |
| 95% CI | |||
| Sensitivity | 103/112 | 91.9% | (85.3% – 96.3%) |
| Specificity | 14/14 | 100% | (76.8% – 100%) |
| Overall | 117/126 | 92.8% | (86.9% – 96.7%) |
The sensitivity of lgM only test is 89.2% (100/112) and specificity is 100% (14/14).
The sensitivity of lgG & IgM test is 91.9% (103/112) and specificity is 100% (14/14).
RT-PCR Method testing was included as clinical site method. Nucleic acid was detected by probe fluorescence PCR. This product contained primers and probes (ORF1ab gene and N gene strains of coronavirus) and internal controls (housekeeping gene beta Globin gene sequences) used in RT-PCR for the in vitro qualitative detection of SARS-CoV-2 RNA in nasopharyngeal swab specimens. The novel coronavirus2019-ncov specific probe was respectively labeled with FAM fluorescence and ROX fluorescence, and the internal standard gene was labeled with VIC fluorescence. The minimum detection limit of the fluorescent RT-PCR assay is 10 copies/reaction.
LIMITATIONS
- Use fresh samples only.
- This test has not been reviewed by the FDA.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus.
- Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. Some specimens containing unusually high titer of heterophile antibodies or rheumatoid factor may affect expected results.
-
Not for the screening of donated blood
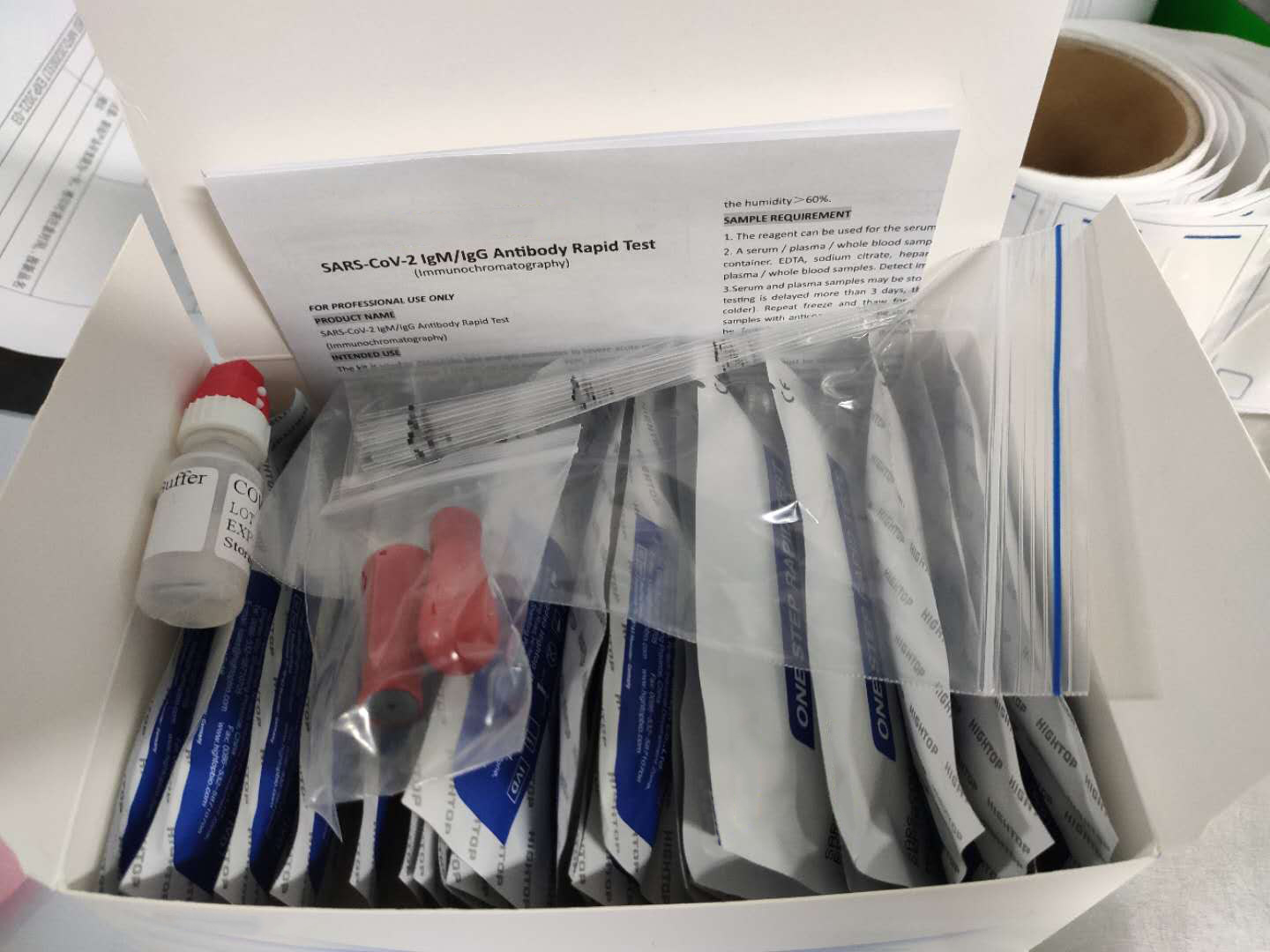
Coronavirus(COVID-19)test
Rapid Test,Coronavirus Test Kit,Coronavirus Test,Covid-19 Test
Dongguan Smart Furniture Co.,Ltd , https://www.smtfurniture.com